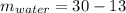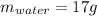Answer:
final temperature will be 0 degree C
Total amount of ice will be
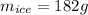
total amount of water
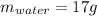
Step-by-step explanation:
After thermal equilibrium is achieved we can say that
Heat given by water = heat absorbed by ice cubes
so we will have
Heat given by water to reach 0 degree C
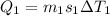
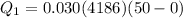
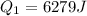
heat absorbed by ice cubes to reach 0 degree
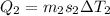
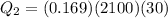
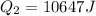
so we will have
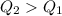
so here we can say that few amount of water will freeze here to balance the heat
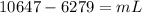
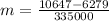
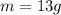
so final temperature will be 0 degree C
Total amount of ice will be
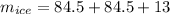
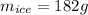
total amount of water