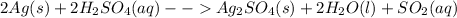Answer:
See the answer below
Step-by-step explanation:
When silver is added to dilute sulphuric acid, no reaction would occur. However, silver reacts with hot and concentrated sulphuric acid to produce silver sulphate, sulphur dioxide, and water.
The equation of the reaction is as below:
silver + sulphuric acid --> silver sulphate + water + sulphur dioxide