Answer:
Final temperature is 295K
Step-by-step explanation:
Where the sample of copper is placed in the water, the heat transferred from the copper is equal that the heat absorbed by the water.
The heat transferred from the copper is:
C×
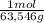 ×mass×ΔT
×mass×ΔT
Where C is molar heat capacity of copper (24,5J/molK)
Mass is 25,0g
And ΔT is final temperature - initial temperature (X-363K)
Also, the heat absorbed by the water is:
-C×
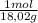 ×mass×ΔT
×mass×ΔT
Where C is molar heat capacity of water (75,2J/molK)
Mass is 100,0g
And ΔT is final temperature - initial temperature (X-293K)
As heat transferred is equal to heat absorbed:
24,5J/molK×
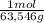 ×25,0g×(X-363K) = -75,2J/molK×
×25,0g×(X-363K) = -75,2J/molK×
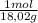 ×100,0g× (X-293K)
×100,0g× (X-293K)
9,64X J/K - 3499J = - 417X J/K + 122273J
426,64X J/K = 125772 J
X = 295K
Final temperature is 295K
I hope it helps!