Answer:
0.507 L or 507 cm³
Step-by-step explanation:
Given,
Initial Volume, V1 = 475 cm³or 0.475 L
Initial temperature, T1 = 25 °C or 298.15 K (since, K = °C + 273.15)
Final temperature, T₂ = 45°C or 318.15 K
We can calculate the new volume;
Using the concept of Charles' law ;
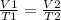
Therefore,
To get, V2, we use;
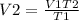


Thus, the gas would expand to a volume of 0.507 L or 507 cm³ to maintain the same pressure.