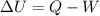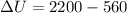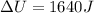Answer:
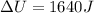
Step-by-step explanation:
As we know by first law of thermodynamics that for ideal gas system we have
Heat given = change in internal energy + Work done
so here we will have
Heat given to the system = 2.2 kJ
Q = 2200 J
also we know that work done by the system is given as

so we have