Answer:
398.3 L
Step-by-step explanation:
Percent composition is percentage by the mass of element present in the compound.
Given that the benzene is
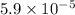 % by mass.
% by mass.
It means that
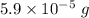 of benzene is present in 100 g of the total sample
of benzene is present in 100 g of the total sample
Also, Given that : Mass of benzene = 235 mg = 0.235 g ( 1 mg = 0.001 g)
Mass % =
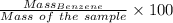
Thus,
Mass of the sample =
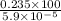 = 398305.08474 g
= 398305.08474 g
Mass of benzene = 0.235 g
Thus, Mass of water = 398304.85 g
Density = 1 g/mL
So, Volume = 398304.85 mL = 398.3 L ( 1 mL = 0.001 L)