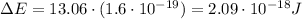Answer:
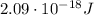
Step-by-step explanation:
The energy levels of a hydrogen atom are given by the formula
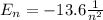 [eV]
[eV]
where n is the number of the level.
Therefore, the energy of the ground state is
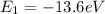
While the energy of the level n = 5 is
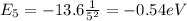
So, the energy that a hydrogen atom needs to excite from n = 1 to n = 5 is
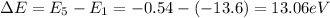
And converting into Joules,