Answer:
It is 4 ions.
Step-by-step explanation:
We know that depression in freezing point of a solution is directly proportional to its molality i.e,
∆
 ∝ m
∝ m
∆
 =
=
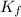 x m
x m
∆
 = (
= (
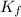 x w x 1000 x i)/(M x W)
x w x 1000 x i)/(M x W)
where ∆
 = depression in freezing point
= depression in freezing point
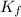 = molal depression freezing point constant or cryscopic constant
= molal depression freezing point constant or cryscopic constant
m = molality of solution,
w = amount of solute ,
M = Molar mass of solute,
W= amount of solvent ,
i = Vant Hoff factor
Given :
m = 4.44g ,
M = 329 ,
W = 100g,
∆
 = 1.1 ,
= 1.1 ,
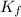 = 1.86 C Kg mol
= 1.86 C Kg mol
Substituting above values in formula,
1.1 = (i x 4.94 x 1000x 1.86)/ (329 x 100)
therefore, i = 3.9386 approx
= 4 ions are present.