Answer:
a) The mass of air in an air filled tire is 8.235 g.
b) The mass of helium in an air filled tire is 1.144 g.
c) 7.091 g is the mass difference between the two.
Step-by-step explanation:
Volume of the tire,V = 860 mL = 0.860 L
Total pressure of the gases in tire,P = 120 psi = 8.16552 atm
1 psi = 0.068046 atm
Temperature of the gases in tire = T = 299.15 K
Total mole of gases = n
PV = nRT (Ideal gas equation)
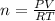
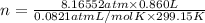
n = 0.2859 mol
a) Mass of air = molar mass of air × 0.2859 mol:
= 28.8 g/mol × 0.2859 mol = 8.235 g
b) Mass of helium = molar mass of helium × 0.2859 mol:
= 4 g/mol × 0.2859 mol = 1.144 g
c) The mass difference between the two gases:
8.235 g - 1.144 g = 7.091 g