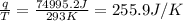Answer:
The entropy change of the surroundings associated with this heat transfer is 255.9J/K
Step-by-step explanation:
The heat evolved by the oxidation of food is 7.2J/Kg.h thus, multiplying the heat by the weight of the person and by the time the total heat (q) is obtained. In this case, the weight is 62.0Kg and a weak has 168 hours.
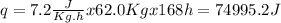
The entropy change of the surrounding associated with this heat transfer can be calculated by the ratio between the heat exchanged and the temperature.
ΔS =