Answer:
1.52 × 10⁻¹⁶ nm
Step-by-step explanation:
We are given,
Energy = 3.36 × 10⁻¹⁷ Joules
We are required to find the wavelength of light
We are going to use,
The formula, E = hλ/c
Where E is the energy, λ is the wavelength, h is the plank's constant and c is the speed of light.
h = 6.626 × 10^-34 J-s
c = 2.998 × 10^8 m/s
Working
From the formula;
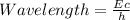
= (3.36 × 10⁻¹⁷ J)(2.998 × 10^8 m/s) ÷ 6.626 × 10^-34 J-s
= 1.52 × 10⁻²⁵ meters
Therefore, the wavelength in m is 1.52 × 10⁻²⁵ m
Conversion
We need to convert m to nm
Conversion factor is 10⁹
Therefore;
1.52 × 10⁻²⁵ m to nm
will be; 1.52 × 10⁻²⁵ m × 10⁹
= 1.52 × 10⁻¹⁶ nm
Thus, the wavelength in nm is 1.52 × 10⁻¹⁶ nm