Answer:
Explanation:
Given that the rate of a reaction is directly proportional to the square of the concentration of A and inversely proportional to concentration of B.
i.e. R = rate of a reaction =
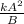
where A shows the concentration of A and B that of B.
If B increases by 100%, then we have B to be replaced by 2B
But R is the same
i.e.
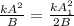 where new A= A1
where new A= A1
Simplify to get
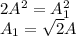
So A increases to sqrt of 2 times original concentration.