Answer:
Mass of rock = 4533.4 kg
Step-by-step explanation:
Percent composition is percentage by the mass of element present in the compound.
Given that the
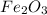 is 82 % by mass.
is 82 % by mass.
It means that 82 kg of
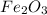 is present in 100 kg of rock
is present in 100 kg of rock
To find the mass of rock which contains 2600 kg of iron
2600 kg = 2600*1000 g
2 moles of iron are present in 1 mole of
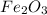
Molar mass of iron = 55.845 g/mol
Mass of 2 moles = 55.845 * 2 = 111.69 g/mol
Molar mass of
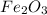 = 159.69 g/mol
= 159.69 g/mol
It means,
111.69 g of iron are present in 159.69 g of
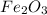
1 g of iron is present in 159.69/111.69 g of
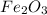
2600*1000 g is present in (159.69/111.69)*2600*1000 g of
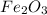
Mass of
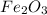 = 3717378.47 g =3717.38 kg
= 3717378.47 g =3717.38 kg
Thus,
82 kg of
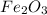 is present in 100 kg of rock
is present in 100 kg of rock
1 kg of
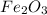 is present in 100/82 kg of rock
is present in 100/82 kg of rock
3717.38 kg of
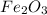 is present in (100/82)*3717.38 kg of rock
is present in (100/82)*3717.38 kg of rock
Mass of rock = 4533.4 kg