Answer:
8,459x10⁻³ M of Cr₂O₇²⁻
Step-by-step explanation:
The equation for the reaction of Fe²⁺ with Cr₂O₇²⁻ is:
Cr₂O₇²⁻(aq) + 6Fe²⁺(aq)+14H₃O⁺(aq) → 2Cr³⁺(aq) + 6Fe³⁺(aq)+21H₂O(l)
The moles of Fe²⁺ that you required for a complete reaction of Cr₂O₇²⁻ are:
0,01515 L ×
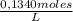 = 2,0301x10⁻³ moles of Fe²⁺
= 2,0301x10⁻³ moles of Fe²⁺
By the equation of the reaction, 1 mol of Cr₂O₇²⁻ reacts with 6 moles of Fe²⁺, thus, moles of Cr₂O₇²⁻ are:
2,0301x10⁻³ moles of Fe²⁺×
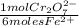 = 3,3835x10⁻⁴ moles of Cr₂O₇²⁻
= 3,3835x10⁻⁴ moles of Cr₂O₇²⁻
The molarity is:
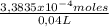 = 8,459x10⁻³ M of Cr₂O₇²⁻
= 8,459x10⁻³ M of Cr₂O₇²⁻
I hope it helps!