Answer:
a) The pH of the solution is 12.13.
b) The pH of the solution is 12.17.
Step-by-step explanation:
Ionic product of water =
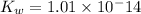
![K_w=[H^+][OH^-]](https://img.qammunity.org/2020/formulas/chemistry/high-school/cgj2x1eylj3d7rly56gh4gh8k95xipvwi4.png)
![1.01* 10^-{14}=[H^+][OH^-]](https://img.qammunity.org/2020/formulas/chemistry/college/rlscaq5pbhzjh0afe9lo3vmsdvawevnno8.png)
Taking negative logarithm on both sides:
![-\log[1.01* 10^-{14}]=(-\log [H^+])+(-\log [OH^-])](https://img.qammunity.org/2020/formulas/chemistry/college/150i3edgqa775a1pzf8e1wurit24xnbkdd.png)
The pH is the negative logarithm of hydrogen ion concentration in solution.
The pOH is the negative logarithm of hydroxide ion concentration in solution.
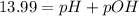
a)
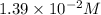 of NaOH.
of NaOH.
Concentration of hydroxide ions:
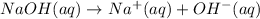
So,
![[OH^-]=1* [NaOH]=1* 1.39* 10^(-2) M=1.39* 10^(-2) M](https://img.qammunity.org/2020/formulas/chemistry/college/tp9qbni66tcwst5ynaoh12bnvie2sf8yes.png)
![pOH=-\log[1.39* 10^(-2) M]=1.86](https://img.qammunity.org/2020/formulas/chemistry/college/9fnmb3orlsbc3ustq08agcwmibhv5akrdd.png)
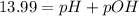
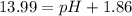
pH=13.99-1.86=12.13
b)
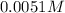 of NaOH.
of NaOH.
Concentration of hydroxide ions:
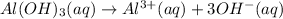
So,
![[OH^-]=3* [Al(OH)_3]=3* 0.0051 M=0.0153 M](https://img.qammunity.org/2020/formulas/chemistry/college/9oybstp4xnpvej2srj1injfrqrvzekp3o1.png)
![pOH=-\log[0.0153 M]=1.82](https://img.qammunity.org/2020/formulas/chemistry/college/3se9gnaom8ft2yj3404g7dgqlxfany4z22.png)
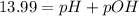
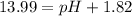
pH=13.99-1.82=12.17