Answer: They follow Einstein's equation, which allows for the conversion of mass to energy.
The statement that is true about nuclear reactions is that they follow Einsteins equation which allows the conversion of mass to energy.
Step-by-step explanation:
Einstein’s mass-energy equation gives the idea that energy and mass are two inter-convertible forms of the same thing. This equation clearly answers the question about the large amount of energy produced by produced in nuclear reaction even though they don’t involve very heavy atoms. It is given by
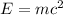
Energy released by a mass is equal to mass times the square of speed of light c.
Value of speed of light
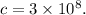
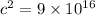 which is a large value.
which is a large value.
Thus high amount of energy can be produced by even a small mass. It is the mass of the reacting species in a nuclear reaction that gets converted to energy and the sum of mass and energy in the overall reaction is always conserved.