Answer:
a) CO₂: 21,9%; CO: 7,0%; CH₄: 18,2%; H₂: 0,8%; N₂: 52,1%
b) 24,09 g/mol
Step-by-step explanation:
a) It is posible to obtain the composition of the gas mixture in weight% using molecular mass of each compound, thus:
12% CO₂×
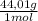 = 528,1 g
= 528,1 g
6% CO×
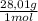 = 168,1 g
= 168,1 g
27,3% CH₄×
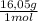 = 438,2 g
= 438,2 g
9,9% H₂×
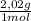 = 20,0 g
= 20,0 g
44,8% N₂×
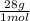 = 1254,4 g
= 1254,4 g
The total mass of the gas mixture is:
528,1g + 168,1g + 438,2g + 20,0g + 1254,4g = 2408,8 g
Thus composition of the gas mixture in weight% is:
CO₂:
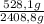 ×100 = 21,9%
×100 = 21,9%
CO:
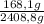 ×100 = 7,0%
×100 = 7,0%
CH₄:
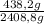 ×100 = 18,2%
×100 = 18,2%
H₂:
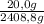 ×100 = 0,8%
×100 = 0,8%
N₂:
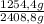 ×100 = 52,1%
×100 = 52,1%
b) The average molecular weight of the gas mixture is determined with mole % composition, thus:
0,12×44,01g/mol + 0,06×28,01g/mol + 0,273×16,05g/mol + 0,099×2,02g/mol + 0,448×28g/mol = 24,09 g/mol
I hope it helps!