Answer:
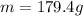 of Ca
of Ca
Step-by-step explanation:
First we are going to write down the balanced reaction:
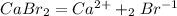
The reduction for the
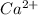 ion will be:
ion will be:
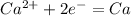
It means that 2 Faraday left for each mol of Ca.
Converting from Faraday to Coulombs:
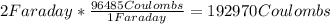
Then we can apply the Faraday´s law:
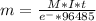
where
m=mass in grams
M=molar mass
I=current
t=time in seconds
e-=number of electrons per mol
Replacing values:
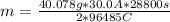
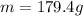 of Ca
of Ca