Answer:
1.2 mol/L
Step-by-step explanation:
The reaction given is:
H₂(g) + I₂(g) ⇄ 2HI (g)
In the begning, the molar concentrations are (number of moles divided by volume):
MH₂ = MI₂ = 3/4 = 0.75 mol/L
So, making a table for the equilibrium:
H₂(g) + I₂(g) ⇄ 2HI (g)
0.75 0.75 0 Initial
-x -x +2x Rects (stoichiometry is 1:1:2)
0.75-x 0.75-x 2x Equilibrium
For a generic reaction aA + bB ⇄ cC +dD, the equilibrium constant is:
![Kc = \frac{{D}^dx[C]^c}{[A]^ax[B]^b}](https://img.qammunity.org/2020/formulas/chemistry/high-school/c6bznyeht3v6bjyc0o5arx5nvlbgj7wdsj.png)
So, for the reaction given:
![Kc = ([HI]^2)/([H2]x[I2])](https://img.qammunity.org/2020/formulas/chemistry/high-school/kilczkv0bp80ntuqml6632cpk4p5hh7kv3.png)
Kc = (2x)²/[(0.75-x)(0.75-x)]
64 = 4x²/(0.5625 - 1.5x + x²)
36 - 96x + 64x² = 4x²
60x² - 96x + 36 = 0 (dividing for 12)
5x² - 8x + 3 = 0
Using Baskhara:
Δ = (-8)² - 4x5x3 = 64 - 60 = 4
x =
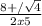
x' = (8+2)/10 = 1
x'' = (8-2)/10 = 0.6
x must be smaller then 0.75, so x = 0.6 mol
Then the molar concentration of HI in equilibrium is 2x = 1.2 mol/L