Answer:
The specific heat capacity for gold in 105 joules which are required to heat 30.0 grams of gold is 0.129 J/(g℃)
Step-by-step explanation:
We make use of the formula
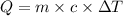
where
∆T = final T - initial T
= 54.9℃ - 27.7℃ = 27.2℃
Q is the heat energy in Joules = 105J
c is the specific heat capacity = ?
m is the mass of Gold = 30.0g
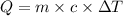
Rearranging the formula
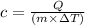
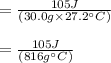
So,
c = 0.129 J/(g℃)
(Answer)