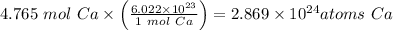The Atoms in 191 g of calcium is
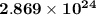 atoms Ca
atoms Ca
Step-by-step explanation:
To calculate the number of atoms of Ca in 191 g Ca.
Convert the given mass of calcium to moles of calcium,
Using its molar mass (referring to a periodic table, this is 40.08gmol):
191g Ca×
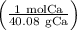 =4.765 mol Ca
=4.765 mol Ca
Using Avogadro's number,
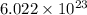 particles mol, calculate the number of atoms present
particles mol, calculate the number of atoms present