Answer:
Thermal speed, v = 2.064 km/s
Step-by-step explanation:
It is given that,
Temperature of Titan's exosphere, T = 172 K
Mass of the hydrogen atom,
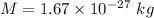
Boltzmann's constant,
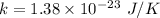
Let v is the thermal speed of Hydrogen. It can be calculated using the following formula as :
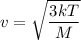
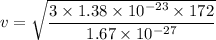
v = 2064.93 m/s
or
v = 2.064 km/s
Hence, the thermal speed of hydrogen in Titan's exosphere is 2.064 km/s.