Answer : The change in internal energy and change in enthalpy of the gas is 1143.2 J/mol and 1600.5 J/mol respectively.
Explanation :
(a) The formula used for change in internal energy of the gas is:
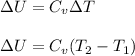
where,
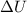 = change in internal energy = ?
= change in internal energy = ?
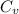 = heat capacity at constant volume =
= heat capacity at constant volume =
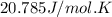
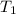 = initial temperature =
= initial temperature =
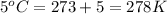
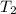 = final temperature =
= final temperature =
Now put all the given values in the above formula, we get:
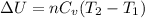
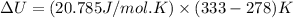
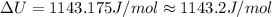
The change in internal energy of the gas is 1143.2 J/mol.
(b) The formula used for change in enthalpy of the gas is:
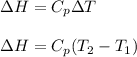
where,
 = change in enthalpy = ?
= change in enthalpy = ?
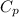 = heat capacity at constant pressure =
= heat capacity at constant pressure =
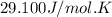
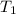 = initial temperature =
= initial temperature =
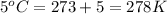
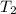 = final temperature =
= final temperature =
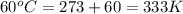
Now put all the given values in the above formula, we get:
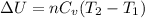
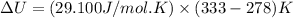
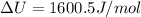
The change in enthalpy of the gas is 1600.5 J/mol.