Answer:
23.0 %
Step-by-step explanation:
Let the gaseous mixture has total 100 moles
Composition of
 = 0.500 %
= 0.500 %
The moles of
 =
=
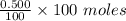 = 0.5 moles
= 0.5 moles
Also,
Molar mass of carbon dioxide = 44.01 g/mol
The formula for the calculation of moles is shown below:

Thus,
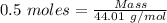
Mass of
 = 22.005 g
= 22.005 g
Composition of air = 99.500 %
The moles of air =
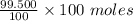 = 99.500 moles
= 99.500 moles
Also,
Given, Average molar mass of air = 29.0 g/mol
The formula for the calculation of moles is shown below:

Thus,
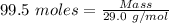
Mass of air = 2885.5 g
Total mass of 100 moles of the mixture = 22.005 g + 2885.5 g = 2907.505 g
Composition of
 = 21 % of air
= 21 % of air
The moles of
 =
=
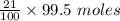 = 20.895 moles
= 20.895 moles
Also,
Molar mass of oxygen = 31.9988 g/mol
The formula for the calculation of moles is shown below:

Thus,
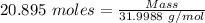
Mass of
 = 668.615 g
= 668.615 g
Thus,
% O by mass =
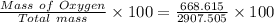 = 23.0 %
= 23.0 %