Answer:
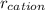 = 0.2162 nm
= 0.2162 nm
Step-by-step explanation:
The force of attraction can be written as:
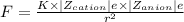
Where,
K is the Coulomb's constant having value 9×10⁹ N. m²/C²
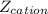 is the charge on the cation
is the charge on the cation
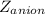 is the charge on the anion
is the charge on the anion
e is electronic charge =
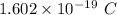
r is the distance between the cation and anion and in bond, it is the sum of the ionic radius of cation and anion
So,
Given, F =
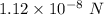
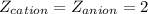
Applying in the equation,
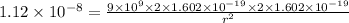
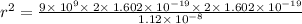
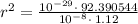
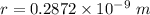
Also, 1 m =
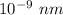
So, r = 0.2872 nm
Also,
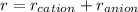
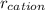 = 0.071 nm
= 0.071 nm
Thus,
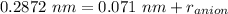
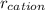 = 0.2162 nm
= 0.2162 nm