Answer:
Wavelength = 486.51 pm
Step-by-step explanation:
The expression for the deBroglie wavelength is:
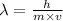
Where,
 is the deBroglie wavelength
is the deBroglie wavelength
h is Plank's constant having value
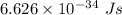
m is the mass of electron having value
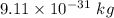
v is the speed of electron.
Given that v = 1495 km/s (Corrected from source)
Also, 1 km = 1000 m
So, v = 1495000 m/s
Applying in the equation as:
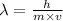
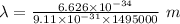
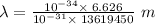
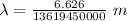
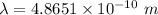
Also, 1 m = 10¹² pm
So, Wavelength = 486.51 pm