Answer: B. mole/s
Step-by-step explanation:
Molar mass is defined as the mass of 1 mole of the compound. It is calculated by adding the masses of all the elements present in the compound.
A mole is defined as the amount of substance that contains Avogardro number of the substance. Avogadro's number is given by
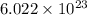 .
.
According to avogadro's law, 1 mole of every substance occupies 22.4 L at STP and contains avogadro's number
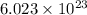 of particles. Standard condition of temperature (STP) is 273 K and atmospheric pressure is 1 atmosphere respectively.
of particles. Standard condition of temperature (STP) is 273 K and atmospheric pressure is 1 atmosphere respectively.
Pressure of the gas is defined as the force exerted by the particles on the walls of the container. It is expressed in various terms like 'mmHg', 'atm', 'kiloPascals' etc..