Answer: The correct answer is Option D.
Step-by-step explanation:
Reduction reaction is defined as the reaction in which a substance gains electrons. Here, the oxidation state of the substance decreases.
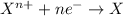
Oxidizing agents are the agents that helps in the oxidation of other substance and itself gets reduced. These agents undergoes reduction reactions.
Oxidation reaction is defined as the reaction in which a substance looses its electrons. Here, oxidation state of the substance increases.
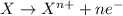
Reducing agents are the agents that helps in the reduction of the other substance and itself gets oxidized. These agents undergoes reduction reactions.
Oxidation state is the number which is given to an atom when it looses or gains electron. It is written as a superscript. In a compound, the total charge is equal to the sum of the charges of all atoms in that compound. For Example: In
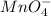 , manganese has +7 oxidation number and oxygen has -2.
, manganese has +7 oxidation number and oxygen has -2.
So, the charge on the compound =
![[=7+(4* (-2))]=-1](https://img.qammunity.org/2020/formulas/chemistry/college/jr28hdsxnz92pmbrzpp9bvkco0mkrncn5a.png)
Hence, the correct answer is Option D.