Answer:
The root mean square speeds of O₂ and UF₆ is 513m/s and 155 m/s respectively.
Solution and Explanation:
- To find how fast molecules or particles of gases move at a particular temperature, the root mean square speed is calculated.
- Root mean square speed of a gas is calculated by using the formula;
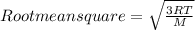
Where R is the molar gas constant, T is the temperature and M is the molar mass of gas in Kg.
Step 1: Root mean square speed from O₂
Molar mass of Oxygen is 32.0 g/mol or 0.032 kg/mol
Temperature = 65 degrees Celsius or 338 K
Molar gas constant = 8.3145 J/k.mol
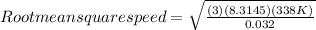
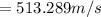
Step 2: Root mean square speed of UF₆
The molar mass of UF₆ is 352 g/mol or 0.352 kg/mol
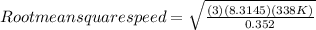
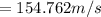
Therefore; the root mean square speeds of O₂ and UF₆ is 513m/s and 155 m/s respectively.