Answer:
Step-by-step explanation:
Hello,
Based on the statement of the equilibrium law:
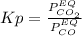
By increasing (doubling) the amount of carbon monoxide, the production of nickel will increase since its amount is directly proportional with its pressure, so, by adding more reactant (in this case carbon monoxide), the equilibrium is rightward shifted based on Le Châtelier's principle even when solid either reactants or products are not contemplated in the equilibrium law.
Best regards.