Answer:
126.73 mL
Step-by-step explanation:
The total pressure of the gas mixture is the sum of the vapor pressure of its constituents. So, the vapor pressure of N₂O(p) can be calculated:
750 = 18.85 + p
p = 750 - 18.85
p = 731.15 torr
It means that for 731.15 torr, N₂O occupied 130 mL. For the general gas equation, we know that
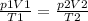
Where p is the pressure, V is the volume, T is the temperature, 1 is the initial state, and 2 the final state. For the same temperatue (21ºC), the equation results on Boyle's law:
p1V1 = p2V2, so:
731.15x130 = 750xV2
750V2 = 95049.5
V2 = 126.73 mL