There will be needed 982.35 mL of solution to obtain 16.1 grams of the salt.There will be needed mL of
Why?
In order to calculate how many milliliters are needed to obtain 16.1 grams of the salt given its concentration, we first need to find its chemical formula which is the following:

Now that we know the chemical formula of the substance, we need to find its molecular mass. We can do it by the following way:
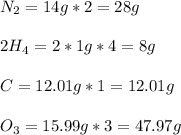
We have that the molecular mass of the substance will be:
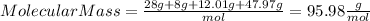
Therefore, knowing the molecular mass of the substance, we need to calculate how many mols represents 16.1 grams of the same substance, we can do it by the following way:
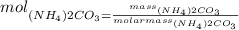
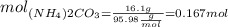
Finally, if we need to calculate how many milliliters are needed, we need to use the following formula:
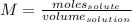
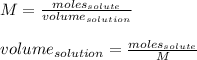
Now, substituting and calculating, we have:
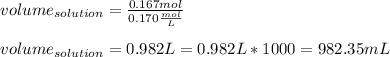
Henc, there will be needed 982.35 mL of solution to obtain 16.1 grams of the salt.
Have a nice day!