The pressure will decrease (from 340kPa to 200kPa) if the volume is increased from 3.2L to 5.44L.
Why?
To solve the problem, we need to pay attention to the system, we can see that the pressure is about to be increased but the temperature does not change, so, it's constant.
Since the temperature is constant, we can use Boyle's Law Equation. Boyle's Law describes the behavior of the pressure and volume with a constant temperature of the ideal gases. According to the Law, if the pressure is increased the volume will decrease, if the pressure decreases, the volume will be increased, and the product between the pressure and volume will be a constant.
We could solve the problem just knowing that the volume has increased, it means that the pressure will decrease.
However, let's prove that:
The expression will be:
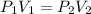
Where,
P1 is the initial pressure.
V1 is the initial volume.
P2 is the final pressure.
V2 is the final volume.
Now, substituting the given information, and calculating, we have:
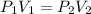
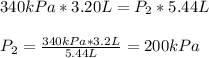
Hence, we can see that the final pressure is lower that the first pressure.
Have a nice day!