Answer:
20, 44, 62
Step-by-step explanation:
To find the number of atoms of each element, we multiply coefficient and subscript
For example
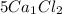 contains
contains
5 × 1 = 5 ,Ca atoms and
5 × 2 = 10, Cl atoms
If there is a bracket in the chemical formula
For example
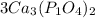
we multiply coefficient × subscript × number outside the bracket to find the number of atoms
(Please note: 3 is the coefficient, and if there is no number given then 1 will be the coefficient )
So
3 × 3 = 9 , Ca atoms
3 × 1 × 2 = 6, P atoms
3 × 4 × 2 = 24, O atoms are present.
So let us find the number of atoms of each element on the left side of the equation
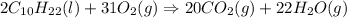
Number of C atoms = 2 × 10 = 20
Number of H atoms = 2 × 22 = 44
Number of O atoms = 31 × 2 = 62
20, 44, 62 are the Answers.