Answer:
0.264 g of
 can be formed from 288 mg of
can be formed from 288 mg of

Step-by-step explanation:
The balanced chemical equation is
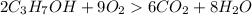
The conversions are
Mass in mg
 is converted to mass in g
is converted to mass in g

Mass in g
 is converted to moles
is converted to moles
 by dividing with molar mass
by dividing with molar mass
Moles
 is converted to moles
is converted to moles
 by using the mole ratio of
by using the mole ratio of
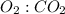 is 9 : 6
is 9 : 6
Moles
 is converted to mass
is converted to mass
 by multiplying with molar mass
by multiplying with molar mass

mass in mg
 > mass in g
> mass in g
 >moles
>moles
 > moles
> moles
 > mass
> mass

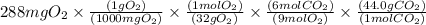
=0.264g (Answer)