Answer:
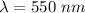
Step-by-step explanation:
The Hi line of the Balmer series is emitted in the transition from n = 3 to n = 2 i.e.
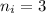 and
and
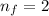
The wavelength of Hi line of the Balmer series is given by :
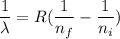
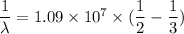
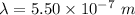
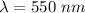
So, the wavelength for this line is 550 nm. Hence, this is the required solution.