Answer:
Radius = 0.8235 cm
Density = 0.7265 g/mL
Step-by-step explanation:
Given that the volume = 2.34 cm³
The volume of the sphere =
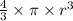
Thus,
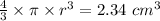
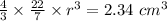
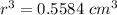
r = 0.8235 cm
Volume = 2.34 cm³ = 2.34 mL (1 cm³ = 1 mL)
Mass = 1.7 g
Density = Mass/ Volume = 1.7 g / 2.34 mL = 0.7265 g/mL