Answer:
The wood was burned 4257 years ago.
Explanation:
The amount of C-14 present can be modeled by the following exponential function.
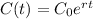
In which C is the amount remaining,
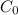 is the initial amount, r is the rate that the amount decrases, and t is the time in years.
is the initial amount, r is the rate that the amount decrases, and t is the time in years.
The C – 14 has a half-life of 5700 years.
This means that
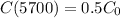
How long ago was the wood burned?
To solve this equation for t when
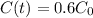 , the first step is finding the rate that the amount decreases. For this, we apply the half-life information in the equation.
, the first step is finding the rate that the amount decreases. For this, we apply the half-life information in the equation.
First step: Find the rate that the amount of C-14 decreases.
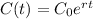
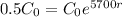
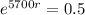
The next step here is applying ln, since ln and e are inverse functions.
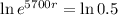
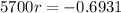
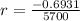
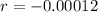
Final step: Find the time
We have that
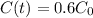 ,
,
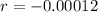 .
.
So:
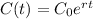
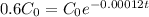
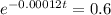
Applying the ln in both sides of the equality.
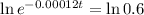
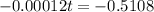
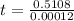
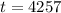
The wood was burned 4257 years ago.