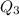Answer:
6700.6 BTU
Explanation:
First you have to warm the block from -15° F to 32°F, the heat to needed to do this is:
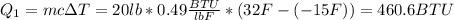
After you need to melt the ice. The heat you need is:
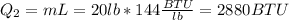
Finally, you need to heat the water from 32° F to 200 ° F, and the heat for this is:
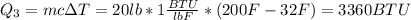
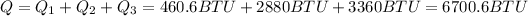 To have the total heat you used you have to su
To have the total heat you used you have to su
 and
and