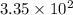Answer : The value of equilibrium constant for this reaction at 262.0 K is
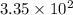
Explanation :
As we know that,
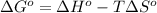
where,
 = standard Gibbs free energy = ?
= standard Gibbs free energy = ?
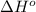 = standard enthalpy = -45.6 kJ = -45600 J
= standard enthalpy = -45.6 kJ = -45600 J
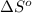 = standard entropy = -125.7 J/K
= standard entropy = -125.7 J/K
T = temperature of reaction = 262.0 K
Now put all the given values in the above formula, we get:
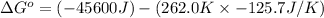
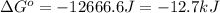
The relation between the equilibrium constant and standard Gibbs free energy is:
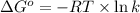
where,
 = standard Gibbs free energy = -12666.6 J
= standard Gibbs free energy = -12666.6 J
R = gas constant = 8.314 J/K.mol
T = temperature = 262.0 K
K = equilibrium constant = ?
Now put all the given values in the above formula, we get:
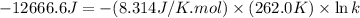
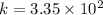
Therefore, the value of equilibrium constant for this reaction at 262.0 K is