Answer:
1,06 g of NaNO₃ and 1,42 g of Na₂SO₄
Step-by-step explanation:
In water, NaNO₃ and Na₂SO₄ dissociates as:
NaNO₃ → Na⁺ + NO₃⁻
Na₂SO₄ → 2 Na⁺ SO₄²⁻
The ionic strength is difined as:
∑
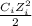 Where Ci and Zi are concentration and charge of each ion in solution. Thus:
Where Ci and Zi are concentration and charge of each ion in solution. Thus:
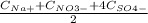 = 0,170 mol/L
= 0,170 mol/L
Knowing
 = 0,130 mol/L you can obtain:
= 0,130 mol/L you can obtain:
0,210 mol/L =
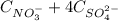 (1)
(1)
The 0,130 mol/L of Na⁺ comes from
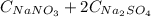 , thus:
, thus:
0,130 mol/L =
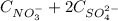 (2)
(2)
Replacing (2) in (1)
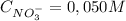
And:
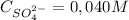
The 0,050 M of NO₃⁻ comes from:
0,050M×0,250L×
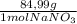 = 1,06 g of NaNO₃
= 1,06 g of NaNO₃
The 0,040 M of SO₄⁻ comes from:
0,040M×0,250L×
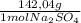 = 1,42 g of Na₂SO₄
= 1,42 g of Na₂SO₄
I hope it helps!