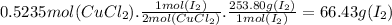Answer:
The mass of I₂ produced is 66.43 g.
Step-by-step explanation:
In a stoichiometry exercise we follow a series of steps.
Step 1: Write the balanced equation
2 CuCl₂ + 4 KI ⇄ 2 CuI + 4 KCl + I₂
Step 2: Look for the relationship between the data we have and the one we are looking for
According to the balanced equation, when 2 moles of CuCl₂ react 1 mole of I₂ is formed. This is the first relation. Secondly, we know that the molar mass of 253.80 g/mol, that is, every mole of I₂ has a mass of 253.80 g. This is the second relation.
Step 3: Apply relations to the data we have