Answer : The mass of sucrose consumed is 156.1 grams.
Explanation :
The expression for first order reaction is:
![[C_t]=[C_o]e^(-kt)](https://img.qammunity.org/2020/formulas/chemistry/college/rft9amygnrxaj7fh268osrn1mx1svyzwy0.png)
where,
![[C_t]](https://img.qammunity.org/2020/formulas/chemistry/college/16n44kmdhlor0i462nohp7yv7f7u48xm7l.png) = concentration of sucrose at time 't'
= concentration of sucrose at time 't'
![[C_o]](https://img.qammunity.org/2020/formulas/chemistry/college/4nzv6ljvpvgub5bmzbof8ig137jttud8kf.png) = concentration of sucrose at time '0' = 0.223 M
= concentration of sucrose at time '0' = 0.223 M
k = rate constant =
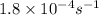
t = time = 282 min = 16920 s (1 min = 60 s)
Now put all the given values in the above expression, we get:
![[C_t]=(0.223)* e^{-(1.8* 10^(-4))* (16920)}](https://img.qammunity.org/2020/formulas/chemistry/college/wx5hq7l89ha5122ymcdfqqrdds9m9axaom.png)
![[C_t]=0.0106M](https://img.qammunity.org/2020/formulas/chemistry/college/55y3r47xxuhhxfht25l51iyssqmvzf61pa.png)
Now we have to calculate the initial and final moles of sucrose.
![n_o=[C_o]* V=0.223M* 2.15L=0.479moles](https://img.qammunity.org/2020/formulas/chemistry/college/euq4uerzw5peogvi70mf930k0o9gov4c7q.png)
![n_t=[C_t]* V=0.0106M* 2.15L=0.0228moles](https://img.qammunity.org/2020/formulas/chemistry/college/2r8n2kvua9ngwri5suzkcrh3zpc9mppx2f.png)
Number of moles of sucrose hydrolysed =

Number of moles of sucrose hydrolysed =
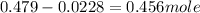
Now we have to calculate the mass of sucrose used.
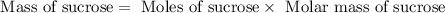
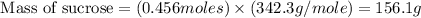
Therefore, the mass of sucrose consumed is 156.1 grams.