Step-by-step explanation:
Non-polar molecules are the molecules which readily dissolve in non-polar solvents.
And, a spontaneous process is defined as the process that will occur on its own without any input of energy from its surroundings.
Therefore, a spontaneous process will always lead to increase in entropy and for a process to be spontaneous at every temperature will have a negative change in enthalpy, that is,
 .
.
As,
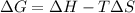
= (-ve) - (-ve)
= -ve
Therefore, we can conclude that free energy will be released when a non-polar molecule is transferred from water to a non-polar solvent.