Answer :
The pressure in torr is, 810.0 torr.
The pressure in atm is, 0.962 atm.
The pressure in atm is, 0.222 atm.
The pressure in pascals is, 102658 Pa.
Explanation :
The conversion used for pressure from kPa to torr is:
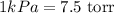
The conversion used for pressure from bar to atm is:
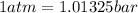
or,
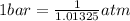
The conversion used for pressure from kPa to atm is:
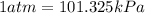
or,
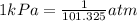
The conversion used for pressure from torr to pascals is:
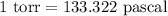
(1) Convert 108 kpa in torr
As,
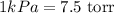
So,
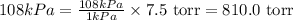
The pressure in torr is, 810.0 torr.
(2) Convert 0.975 bar in atmospheres
As,
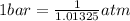
So,
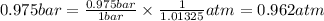
The pressure in atm is, 0.962 atm.
(3) Convert 22.5 kpa in atmospheres
As,
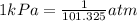
So,
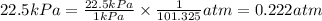
The pressure in atm is, 0.222 atm.
(4) Convert 770 torr in pascals
As,
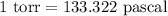
So,
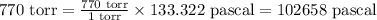
The pressure in pascals is, 102658 Pa.