Answer : The correct option is, x = 10
Explanation : Given,
Concentration of
 ion = 0.001 M
ion = 0.001 M
pH : It is defined as the negative logarithm of hydrogen ion or hydronium ion concentration.
First we have to calculate the pH.
![pH=-\log [H^+]](https://img.qammunity.org/2020/formulas/chemistry/high-school/ipfjz05f4cfbguiwup37xvxa7furlbuapf.png)
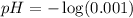
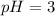
Now we have to calculate we the value of 'x'.
The given expression is:
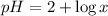
Now put the value of pH in this expression, we get:
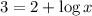
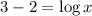
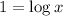
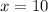
Therefore, the value of 'x' is 10.