Step-by-step explanation:
As it is known that non-electrolytes do not dissociate. Therefore, molarity of such a solution is equal to the osmolarity of solution.
As, molar mass of ethanol = 46.07 g/mol
Therefore, no. of moles of ethanol will be calculated as follows.
No. of moles =
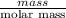
=
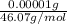
=
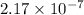 mol
mol
As, molarity is moles of solute in liter of solution. Hence, molarity of ethanol is as follows.
Molarity =
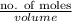
=
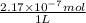
=
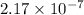 mol/L
mol/L
Since, for the given solution Molarity = osmolarity
Thus, we can conclude that osmolarity of .00001 grams (0.1 mg%) of ethanol in 1 L is
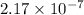 osmol/L.
osmol/L.