Answer : The mass of
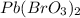 formed is 164.4 grams.
formed is 164.4 grams.
Explanation :
The balanced chemical reaction will be,
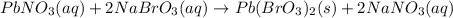
First we have to calculate the moles of
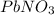 .
.
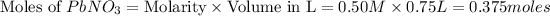
From the balanced chemical reaction we conclude that,
Moles of
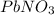 = Moles of
= Moles of
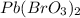 = 0.375 mole
= 0.375 mole
The moles of precipitate formed
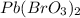 = 0.375 mole
= 0.375 mole
Now we have to calculate the moles of
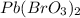 that dissolve in
that dissolve in
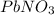 .
.
The dissociation of lead bromate is written as:
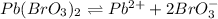
The expression for solubility constant for this reaction will be,
![K_(sp)=[Pb^(2+)][BrO_3^-]^2](https://img.qammunity.org/2020/formulas/chemistry/college/3cq3w24yv73uzrc0ih3a012qyphhy3svm9.png)
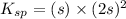
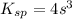
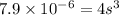
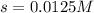
Total volume of the solution = 0.75 + 0.850 = 1.60 L
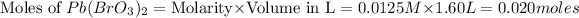
The moles of
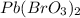 that dissolve in
that dissolve in
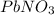 = 0.020 mole
= 0.020 mole
The moles of precipitate formed
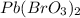 = 0.375 - 0.020 = 0.355 mole
= 0.375 - 0.020 = 0.355 mole
Now we have to calculate the mass of
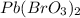 formed.
formed.
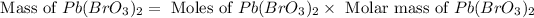
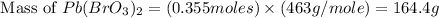
Therefore, the mass of
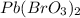 formed is 164.4 grams.
formed is 164.4 grams.