Answer:
32,127.02 grams of hydrogen hexafluorosilicate will contain this 25,434 grams of F.
Step-by-step explanation:
Volume of cylindrical reservoir = V
Radius of the cylindrical reservoir = r = d/2
d = diameter of the cylindrical reservoir = d =

r = d/2 = 22.5 m
Depth of the reservoir = h = 10.0 m
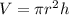


Volume of water cylindrical reservoir : V
Density of water,d = 1 kg/L
Mass of water cylindrical reservoir = m

1.6 kilogram of fluorine per million kilograms of water. (Given)
Concentration of fluorine in water = 1.6 kg/ 1000,000 kg of water
In 1000,000 kg of water = 1.6 kg of fluorine
Then
 of water have x mass of fluorine:
of water have x mass of fluorine:


15,896,250 kg water of contains mass 25.434 kg of fluorine.
25.434 kg = 25434 g
25,434 grams of fluorine should be added to give 1.6 ppm.
Percentage of fluorine in hydrogen hexafluorosilicate :
Molar mass hydrogen hexafluorosilicate = 144 g/mol

Total mass of hydrogen hexafluorosilicate = m'

m' = 32,127.02 g
32,127.02 grams of hydrogen hexafluorosilicate will contain this 25,434 grams of F.