Step-by-step explanation:
The given data is as follows.
Refractive index of mixture = 1.456
Refractive index of hexane = 1.375
Refractive index of toulene = 1.497
Let mole fraction of hexane =
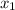
and, mole fraction of toulene =
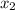
Also,
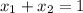
or,
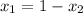
Hence, calculate the mole fraction of hexane as follows.
refractive index mixture= mole fraction hexane × ref index hexane + mole fraction toluene × ref index toluene.
1.456 =
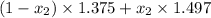
1.456 =
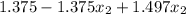
0.081 =
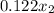
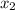 =
=
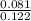
= 0.66
Since,
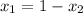
= 1 - 0.66
= 0.34
Thus, we can conclude that mole fraction of hexane in your sample is 0.34.